Burundi and social media rumors, how COVID-19 vaccine testing works?
Briefing Issue 68, Tuesday, April 7, 2020
This is the online version of RegionWeek for decision-makers and young professionals, a newsletter about depth and context to Burundi and East Africa stories— written by me, Fabrice Iranzi. You can sign up for your own subscription to get concise information about game-changing events and insights straight to your inbox:

Dear RegionWeek Reader,
Last week, rumors invaded social media tabulating Burundi among countries to host experiments on coronavirus vaccine. Burundi government refutes being listed among countries to test the COVID-19 vaccine.
This followed a French doctor uttering on TV that the vaccine should be taken to Africa for experiments.
The debate between the two French doctors, Camille Locht from INSERM (French National Institute of Health and Medical Research) and Jean-Paul Mira, head of intensive medicine and the intensive care unit at Paris' Cochin hospital, is available to watch on YouTube.
It rose wrath in African superstars Didier Drogba and Samuel Eto’o strongly condemned the “racist rhetoric”.
The Burundi Ministry of Health denied being involved in any COVID-19 vaccine testing in Burundi, assured the spokesperson of the ministry of health Mr. J. Bosco Girukwishaka in a press conference this Tuesday, April 7th, 2020. He refuted the allegations of “Burundi receiving vaccine from China”

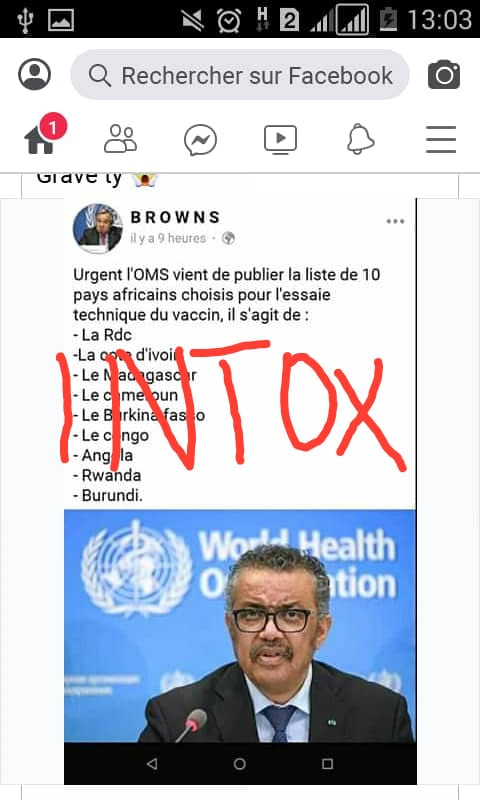
This Tuesday, the WHO Director Tedros Adhanom Ghebreyesus said that “Africa can’t and won’t be a testing ground for any vaccine”
"We will use the same protocol and if there is a need to be tested it is well to treat human beings equally. The hangover from a colonial mentality has to stop. WHO will not allow this to happen."
He added that the comments were "a disgrace" and "appalling to hear in the 21st century from scientists".
Who is working on COVID19 Vaccines?
Two companies appear to be in the lead right now. In March, Moderna initiated the first clinical testing in humans of an experimental COVID-19 vaccine. The biotech's messenger RNA (mRNA) vaccine was developed in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID). Inovio announced on April 6 that it had begun a phase 1 clinical study of experimental COVID-19 DNA vaccine INO-4800.
Here are all the companies:

How Vaccine Development and Testing work?
The current system for developing, testing, and regulating vaccines was developed during the 20th century as the groups involved standardized their procedures and regulations.
In the European Union, the European Medicines Agency supervises the regulation of vaccines and other drugs. A committee of the World Health Organization makes recommendations for biological products used internationally. Many countries have adopted the WHO standards.
Vaccine development is a long, complex process, often lasting 10-15 years and involving a combination of public and private involvement.
Here are the key steps to get the vaccine approved to be used
Step 1: Laboratory and Animal Studies
This is an exploratory stage, academic and governmental scientists identify natural or synthetic antigens that might help prevent or treat a disease. They conduct pre-clinical studies, use tissue-culture or cell-culture systems and animal testing to assess the safety of the candidate vaccine and its immunogenicity, or ability to provoke an immune response. Animal subjects may include mice and monkeys. At this step, a sponsor, or usually a private company, submits an application for an Investigational New Drug.
Step 2: Clinical Studies with Human Subjects
This stage is used to assess the safety of the candidate vaccine and to determine the type and extent of the immune response that the vaccine provokes.
This first attempt to assess the candidate vaccine in humans involves a small group of adults, usually between 20-80 subjects. If the vaccine is intended for children, researchers will first test adults, and then gradually step down the age of the test subjects until they reach their target.
A larger group of several hundred individuals participates in Phase II testing to study the candidate vaccine’s safety, immunogenicity, proposed doses, schedule of immunizations, and method of delivery.
Successful Phase II candidate vaccines move on to larger trials, involving thousands to tens of thousands of people.
The Phase III tests are randomized and double-blind and involve the experimental vaccine being tested against a placebo (the placebo may be a saline solution, a vaccine for another disease, or some other substance).
Step 3: Approval and Licensure
After a successful Phase III trial, the vaccine developer will submit a Biologics License Application then the factory where the vaccine will be made is inspected and the labeling of the vaccine is approved.
After licensure, the production of the vaccine, (including inspecting facilities and reviewing the manufacturer’s tests of lots of vaccines for potency, safety, and purity ) is monitored continuously.
A variety of systems monitor vaccines after they have been approved. They include Phase IV trials, the Vaccine Adverse Event Reporting System, and the Vaccine Safety Datalink.
In general, vaccines are more thoroughly tested than non-vaccine drugs because the number of human subjects in vaccine clinical trials is usually greater.
Thanks for reading!
All the best to you and your colleagues, friends, and families during a challenging and unsettling time.
Please feel free to reply to this email and tell us what you like or don't like, this helps us evolve and improve as we go.
Fabrice Iranzi
Editor, RegionWeek.com
If you wish to support this Newsletter we created a $5/Month subscription plan, you can join our Premium community of supporters by clicking here
Ressources and notes
https://www.historyofvaccines.org/
RegionWeek is a Burundi-based media for a new generation of achievers in Africa, a platform devoted to chronicling the journey to Freedom and Empowerment.



